Abstract
Introduction: As a newly approved third line therapy for hematologic malignancies, chimeric antigen receptor (CAR)-T cell therapy can provide patients with a life-saving therapeutic option. CAR T-cell therapy carries risks of toxicities and potentially intensive health care use. However, given its relatively recent approval (approved in 2017), there is limited real world data on healthcare utilization after CAR-T cell infusion. We examined hospitalizations and ED visits for the first 12 mo after CAR-T cell infusion.
Methods: We used Truven Health MarketScan database (one of the largest private health insurance claims datasets in the US) to identify commercially insured patients who received CAR-T cell infusion between Jan 2017 and Dec 2019 at age <65y. CAR-T cell therapy was identified using ICD-10 codes (Q2042, Q2041, 0540T, XW033C3, XW043C3) and national drug codes for axicabtagene ciloleucel and tisagenlecleucel. Patients were followed for hospitalizations and/or ED visits from CAR-T cell infusion to death, loss of coverage, 12 mo or 12/31/2019 (end of available data), whichever came first. The cumulative incidence of first hospitalization from CAR-T cell therapy was calculated using competing risks method. We also examined cumulative cause-specific hospitalizations per person-month at 30d, 31-90d, and >90d from infusion. Total number of ED visits and reasons for ED visits were examined by primary disease and by time from CAR-T cell infusion.
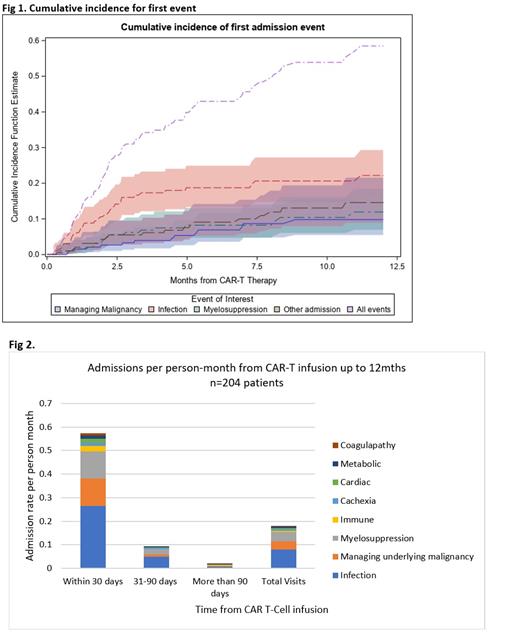
Results: Overall, 204 patients (66% male) received CAR-T cell infusions (96% tisagenlecleucel) over the 3y period; median age at 1 st infusion was 55y (range 1-64). Cancer diagnoses included diffuse large B cell lymphoma (DLBCL, 62.8%), chronic lymphocytic leukemia (CLL, 12.3%), multiple myeloma (MM, 9.3%), other (15.6%). Median follow-up from CAR-T cell infusion was 3.2 mo (0-12); 11% were followed for all 12 mo. Hospitalizations: Ninety-one patients (44%) had 191 hospitalizations within 12 mo of CAR-T infusion (n=1: 47%, n=2: 24%, n >2: 28.5%); 16% of all CLL patients 12% of all DLBCL and 11% of all MM patients had >2 hospitalizations. Most prevalent reasons for hospitalization included infection (n=79; 41.3% of hospitalizations), myelosuppression (n=38; 19.9%), and management of underlying malignancy (n=35; 18.3%). The average length of stay per hospitalization was highest for infection (13.5d), followed by management of underlying malignancy (8.3d), and myelosuppression (5.9d). The cumulative incidence at 12 mo for cause-specific hospitalizations included: infection (22.2%), myelosuppression (11.9%), and managing malignancy (9.8%) (Fig 1). Overall, this cohort experienced 0.2 hospitalizations/person-mo. The majority of these hospitalizations occurred within the 1 st mo following CAR-T cell therapy (0.6/person-mo), declining to 0.11/person-mo at 31-90d, and to 0.04/person-mo at >90d (Fig 2). Infection and myelosuppression remained the most common reasons for hospitalizations across the three time periods. There were no statistically significant factors predicting hospitalization in multivariable models. ED visits: Fifty patients (25%) had 71 ED visits after CAR-T cell infusion (n=1: 76%; n=2: 14%; n>2: 10%). Reasons for ED visits included infections (n=14, 19.7%), myelosuppression (n=11, 15.5%), and cardiac emergencies (n=6, 8.5%). Median time to ED was 107d (range 8-355d). Nine ED visits occurred within the 1 st mo after CAR-T cell infusion (infection, epigastric/chest pain, thrombosis/hemorrhage [n=2 each], neutropenia, altered mental status, primary disease [n=1 each]).
Conclusion: Real world data show that over the first 12 mo. after CAR-T cell therapy, 44% of the patients are re-hospitalized one or more times and 25% are seen in the emergency room. Infection is the most common reason for unplanned visits (hospitalizations or ED visits), followed by myelosuppression. The probability of these hospitalizations is highest within the first month, declining rapidly thereafter. These findings can be used to inform management strategies to mitigate unplanned healthcare utilization.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal